Chemical polarity
- "Polar molecule" and "Non-polar" redirect here. For other uses see Polar (disambiguation))

In chemistry, polarity refers to a separation of electric charge leading to a molecule having an electric dipole. Polar molecules can bond together due to dipole–dipole intermolecular forces between one molecule (or part of a large molecule) with asymmetrical charge distribution and another molecule also with asymmetrical charge distribution. Molecular polarity is dependent on the difference in electronegativity between atoms in a compound and the asymmetry of the compound's structure. For example, a molecule of water is polar because of the unequal sharing of its electrons in a "bent" structure, whereas methane is considered non-polar because the carbon shares the electrons with the hydrogen atoms uniformly. Polarity underlies a number of physical properties including surface tension, solubility, and melting- and boiling-points.
Contents |
Theory

Electrons are not always shared equally between two bonding atoms: one atom might exert more of a force on the electron cloud than the other. This "pull" is termed electronegativity and measures the attraction for electrons a particular atom has. The unequal sharing of electrons within a bond leads to the formation of an electric dipole: a separation of positive and negative electric charge. Fractional charges are denoted as δ+ (delta plus) and δ− (delta minus). These symbols were introduced by Christopher Ingold and his wife Dr. Hilda Usherwood in 1926.[1]
Atoms with high electronegativities — such as fluorine, oxygen, and nitrogen — exert a greater pull on electrons than atoms with lower electronegativities. In a bonding situation this can lead to unequal sharing of electrons between atoms, as electrons will spend more time closer to the atom with the higher electronegativity.
Bonds can fall between one of two extremes — being completely non-polar or completely polar. A completely non-polar bond occurs when the electronegativities are identical and therefore possess a difference of zero. A completely polar bond is more correctly termed ionic bonding and occurs when the difference between electronegativities is large enough that one atom takes an electron from the other. The terms "polar" and "non-polar" bonds usually refer to covalent bonds. To determine the polarity of a covalent bond using numerical means, the difference between the electronegativity of the atoms is taken. If the result is between 0.4 and 1.7 then, generally, the bond is polar covalent.
Polarity of molecules
A molecule is composed of one or more chemical bonds (covalent bonds) between molecular orbitals of different atoms. A molecule may be polar either as a result of polar bonds due to differences in electronegative as described above, or as a result of an asymmetric arrangement of non-polar covalent bonds and non-bonding pairs of electrons known as a full molecular orbital.
- Example 1. The hydrogen fluoride, HF, molecule is polar by virtue of polar covalent bonds — in the covalent bond electrons are displaced towards the more electronegative fluorine atom.
- Example 2. In the ammonia, NH3, molecule the three N–H bonds have only a slight polarity (towards the more electronegative nitrogen atom). However, the molecule has two lone electrons in an orbital, that points towards the fourth apex of the approximate tetrahedron, (VSEPR). This orbital is not participating in covalent bonding; it is electron rich which results in a powerful dipole across the whole ammonia molecule.
- Example 2.5. In the ozone, O3, molecule the two O–O bonds are non-polar (there is no electronegativity difference between atoms of the same element). However, the distribution of other electrons is uneven — since the central atom has to share electrons with two other atoms, but each of the outer atoms only have to share electrons with one other atom, the central atom is more deprived of electrons than the others (the central atom has a formal charge of +1, while the outer atoms each have a formal charge of −1/2). Since the molecule has a bent geometry, this results in a dipole across the whole ozone molecule.
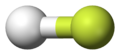 Hydrogen fluoride, the more electronegative fluoride atom is shown in yellow |
 Hydrogen fluoride, red represents partially negatively charged regions |
 Ammonia, the two lone electrons are shown in yellow, the hydrogen atoms in white |
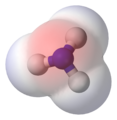 Ammonia, red represents partially negatively charged regions |
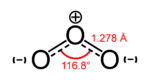 Ozone |
In a similar manner, a molecule may be non-polar either because there is (almost) no polarity in the bonds or because of the symmetrical arrangement of polar bonds.
- Example 3. In the methane, CH4 molecule the four C–H bonds are arranged tetrahedrally around the carbon atom. Each bond has polarity (though not very strong). However, the bonds are arranged symmetrically so there is no overall dipole in the molecule.
- Example 4. The boron trifluoride, BF3, molecule has a trigonal planar arrangement of three polar bonds at 120o This results in no overall dipole in the molecule.
- Example 5. The oxygen, O2, molecule does not have polarity in the covalent bond because of equal electronegativity, hence there is no polarity in the molecule.
 Methane, the bonds are arranged symmetrically so there is no overall dipole |
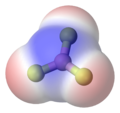 Boron trifluoride, trigonal planar arrangement of three polar bonds results in no overall dipole |
Predicting molecule polarity
| Formula | Description | Example | |
| Polar | AB | Linear Molecules | CO |
|---|---|---|---|
| HAx | Molecules with a single H | HF | |
| AxOH | Molecules with an OH at one end | C2H5OH | |
| OxAy | Molecules with an O at one end | H2O | |
| NxAy | Molecules with an N at one end | NH3 | |
| Non-polar | A2 | Diatomic molecules of the same element | O2 |
| CxAy | Most carbon compounds | CO2 |
Properties and examples
While the molecules can be described as "polar covalent", "non-polar covalent", or "ionic", it must be noted that this is often a relative term, with one molecule simply being more polar or more non-polar than another. However, the following properties are typical of such molecules.
Polar molecules
Examples of common household polar molecules include sugar, for instance the sucrose sugar variety. Sugars have many polar oxygen–hydrogen (-OH) groups and are overall highly polar.
Due to the polar nature of the water molecule (H2O) itself, polar molecules are generally able to dissolve in water.
 Sucrose, a sugar, has many polar -OH groups |
 Water is a polar solvent |
Non-polar molecules
A non-polar compound occurs when there is an equal sharing of electrons between two different atoms. Examples of household non-polar compounds include fats, oil and petrol/gasoline. Therefore (per the "oil and water" rule of thumb), most non-polar molecules are water insoluble (hydrophobic) at room temperature. However many non-polar organic solvents, such as turpentine, are able to dissolve polar substances. When comparing a polar and non-polar molecule with similar molar masses, the polar molecule generally has a higher boiling point, because of the dipole–dipole interaction between their molecules. The most common form of such an interaction is the hydrogen bond, which is also known as the H-bond.
Hybrids
Large molecules that have one end with polar groups attached and another end with non-polar groups are good surfactants. They can aid in the formation of stable emulsions, or blends, of water and fats. Surfactants reduce the interfacial tension between oil and water by adsorbing at the liquid–liquid interface.
|
This complex molecule has several polar groups (hydrophilic — water loving) on the right side and a long non-polar chain (lipophilic — fat loving) at the left side. This gives it surfactant properties |
 A micelle — the lipophilic ends of the surfactant molecules dissolve in the oil, while the hydrophilic charged ends remain outside in the water phase, shielding the rest of the hydrophobic micelle. In this way the small oil droplet becomes water soluble. |
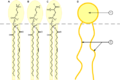 Phospholipids are effective natural surfactants that have important biological functions |
 Cross section view of the structures that can be formed by phospholipids. They can form a micelle and are a vital in forming cell membranes |
See also
- Bond dipole moment
- Solubility
- Colloid
- Detergent
- Dipole
- Covalent bond
- Electronegativity
- Dielectric
- Chemical bonding
References
|
||||||||||||||
|
||||||||||||||||||||||||